Sample collection
The bacterial samples were collected from agricultural fields near the Ranga Reddy district, Telangana, India (“17.3891 N 77.8367 E”). The sampling site was rich in flora and consisted of diverse agricultural plant species. A soil probe or trowel was utilized to collect the rhizosphere soil samples at varying depths. Subsequently, numerous soil sub-samples were prepared, added to a larger container, and mixed thoroughly to make a homogenous mixture. After mixing, a small amount (~ 300 mg) of the prepared homogenous mixture was added to 50 mL sterilized, self-sealed, and labeled Falcon tubes. The sterilized Falcon tubes were initially kept at ambient temperature and then at 4 °C. The temperature was maintained until the next step of analysis25,26.
Isolation of the microbial consortia
After field sampling, the collected bacterial consortia were isolated, 90 mL of peptone saline solution (0.1% peptone (w/v), 0.85% NaCl) and 10 g of the homogenized soil sample were suspended, incubated in an orbital shaker for 1 h at 30 °C at 150 rpm, and then allowed to stand for 30 min. After incubation, 1mL of the aliquot was taken and added to 99 mL of nutrient broth (g/L): 5U supplemented with 5 U of peptone, 1 U of beef/yeast extract, 15 U of agar, 5 U of NaCl and distilled water (HiMedia, India). 4 U of nystatin antifungal agent was added to prevent fungal growth and the solution was then re-incubated for 3–4 days at 30 °C at 150 rpm. After incubation, suspensions were serially diluted (10−1 to 10−8). 100 µL from each dilution was spread plated onto the nutrient agar plates using a L-shaped sterilized spreader. The plates were re-incubated at 30 °C for 48 h. Distinct bacterial colonies were chosen and subjected to subculturing in fresh nutrient media. Pure cultures were stored at 4 °C. The concentration of the bacterial consortia was obtained using a UV/Vis spectrophotometer (Optical Density (OD) at A600) (Thermo Fisher Scientific)6,27,28.
Standardization and preparation of the inoculum to produce NPs
For the standardization procedure, a 0.5 McFarland solution was prepared by mixing 99.5 mL of 0.18 mol/L (1% v/v) of sulfuric acid (H2SO4) and 0.5 mL of 0.048 mol/L (1.1750% w/v) of dehydrated barium chloride (BaCl2) solution. Subsequently, the turbidity of the standardized solution was calculated in different test tubes. The absorbance of 0.5 McFarland solution was computed at OD – 600 nm (A600) (0.8–1.0) (Thermo Fisher Scientific). To prevent evaporation and concentration loss due to light, the standardized solution was kept in an airtight container at room temperature (RT). Before comparing the bacterial suspension, the turbidity standard tube was thoroughly mixed using a vortex to obtain a consistent turbid appearance. After overnight incubation, a 5 mL (0.5 McFarland) bacterial culture was suspended in a nutrient broth and incubated for 4 h at 37 °C. The turbidity was regulated by employing a sterile cotton swab. The inoculum was distributed uniformly in the entire agar medium by swirling the plate at an angle of 60°28,29.
Screening of the bacterial isolates to produce NPs
Primary screening
The production of NPs was assessed by determining the potential antagonistic activity of the selected isolates. The primary screening was conducted against the test bacterial strains by employing a transverse pattern on nutrient agar plates. The selected bacterial strains were streaked horizontally and incubated for 24–36 h at 30 °C. After incubation, the test microorganisms (Escherichia coli (MTCC 739) and Bacillus subtilis (MTCC 121)) were streaked perpendicularly (at 90 °) to the screened isolates. The plates were re-incubated for 24–48 h at 30 °C. The antagonistic activity was determined by observing the inhibitory or lytic activity at the intersecting regions. The bacterial isolates that gave affirmative results were selected for secondary screening to validate their bioactivity. All the experiments were conducted in triplicate. Their outcomes were assessed in the form of mean ± standard error mean (SEM)30,31.
Seed overlay method/crowd plate technique
The bacterial isolates displaying antagonistic activity in primary screening were subjected to the seed overlay assay. The isolates were spot-inoculated using a sterile metal needle onto a rich nutrient agar plate and incubated for 48 h at 30 °C. After incubation, 2 mL of chloroform was added to each plate to stop the bacterial growth and allow production of secondary metabolites only. The plates were re-incubated for 1 h at RT. 100 µL of test pathogenic bacteria (Bacillus subtilis (MTCC 121)) was mixed thoroughly with 2 mL of chilled nutrient broth containing 0.6% agar and spread uniformly over treated plates and incubated for 24 h. The antagonistic activity was determined by calculating the zone of inhibition (ZOI), suggesting the effective production of bioactive compounds. All the experiments were conducted in triplicate. Their outcomes were assessed in the form of mean ± standard error mean (SEM)31,32.
Secondary screening
The bacterial strains that demonstrated positive results in the preceding assays were further selected for secondary screening by using the agar well diffusion method. All the active bacterial strains were cultured in the nutrient broth at 30 °C and 150 rpm for 24 h. After incubation, the cultures were adjusted to procure an OD-600 nm (A600) ~ 0.8 using a UV/Visible Spectrophotometer (Thermo Fisher Scientific). The cultures were centrifuged for 10 min at 5000 x g to obtain a cell-free crude extract, then stored at 4 °C.
The test pathogen (Staphylococcus aureus (MTCC 96)) was swabbed onto Muller Hinton (MH) agar (HiMedia, India) plates to generate a carpet texture using a sterile cotton swab. After swabbing, five wells (6 mm diameter and 10 mm deep) were constructed on the agar plate using a sterile cork borer. Each well was loaded with 100 µL of the crude extract at different concentrations: C1–100%, C2–50%, and C3–25%. An antibiotic disc (ciprofloxacin) at 100 ppm was used as the positive control, while distilled water was used as the negative control. The plates were allowed to rest for 1–2 h and incubated for 24 h at 37 °C, and then their ZOIs were measured. All the experiments were conducted in triplicate. Their outcomes were assessed in the form of mean ± standard error mean (SEM)24,24.
Determination of MBC and MIC
The Minimum Bacterial Concentration (MBC) and Minimum Inhibitory Concentration (MIC) of the subsequent bacterial strains were determined from the cell-free crude extract against the test pathogen (Staphylococcus aureus (MTCC 96)). The secondary metabolites obtained from secondary screening were extracted and concentrated in a dissolved nutrient broth. A two-fold serial dilution of the extract was made at varying concentrations (100%, 50%, 25%, 12.5%, 6.25%, 3.125%, and 1.562%). 0.1 mL of the standardized test inoculum and 0.1 mL of cell extract were added to all eight test tubes. Additionally, 0.1 mL of distilled water was added as a negative control in the remaining test tube. All the test tubes were incubated for 18–24 h at 37 °C. After incubation, the MIC was determined by observing the test tubes with no visible turbidity. 0.1 mL solution was taken from these test tubes and plated onto MH agar (HiMedia, India) and incubated for 24 h at 37 °C. Following incubation, MBC was assessed by studying the presence or absence of bacterial colonies. All the experiments were conducted in triplicate. Their outcomes were assessed in the form of mean ± standard error mean (SEM)24,33.
Antibiotic susceptibility testing (AST)
Antibiotic Susceptibility Testing (AST) was conducted, employing the disk diffusion method. The selected bacterial cultures were swabbed onto MH agar plates (HiMedia, India) using a sterile cotton swab to make a carpet texture. The plates were then incubated for 10 min. After equal spreading, the selected isolates were tested for their activity against six antimicrobial agents at various concentrations: amoxicillin (10 µg), cefotaxime (30 µg), ampicillin (10 µg), ciprofloxacin (5 µg), amikacin (30 µg), and erythromycin (15 µg). The plates were then sealed and incubated for 24 h at 37 °C. Antagonistic behavior was determined by measuring the ZOI. All the experiments were conducted in triplicate. Their outcomes were assessed in the form of mean ± standard error mean (SEM)33,34.
Biochemical and morphological screening
The examined bacterial isolates were stored in nutrient slants at 4 °C. Primary identification was done by determining the cell morphology characteristics, colony formation, and Gram staining. A series of biochemical tests were conducted to identify secondary identification of bacterial strains. The observed bacterial characteristics were compared with Bergey’s Manual of Determinative Bacteriology as a reference. A temperature of 37 °C was maintained for all the biochemical tests35.
gDNA isolation, purification, and PCR amplification
After a series of screening procedures, the genomic DNA (gDNA) was extracted, employing the phenol/chloroform method. For verifying the purity and integrity of the bacterial strains, the extracted gDNA was run on 1% agarose gels in 1x TAE buffer (Tris-acetate-EDTA: pH 8.3, 89 mM Tris base, 89 mM boric acid, and 2 mM EDTA in 1 L of water) at 10 V mm-1 for 90 min. The results were inspected under a UV transilluminator and recorded using Bio-Rad’s Gel Doc XR. The samples were amplified using GeneJET PCR Purification and Gel Extraction Kit (Thermo Fisher Scientific), which contained a master mix of 50 µL: 1 µL of Taq DNA polymerase enzyme (3U/mL), 1 µL of gDNA template, 10X Polymerase assay buffer, 4 µL of deoxy-nucleoside triphosphates dNTPs (2.5mM each), 2 µL of universal forward and reverse universal primers (each), 1X of gel loading buffer, and 30 µL of water. The amplified products were run for 30 cycles. The gDNA was stored at 4 °C. PCR product was obtained and reloaded onto the 1% agarose gels against the 500 bp ladder for gDNA detection32,36,37,38,39.
16S rRNA sequencing
The amplified and eluted gene products were further subjected to chain termination sequencing (16S rRNA sequencing; ribotyping) to identify the specific bacterial 16S rRNA gene products. After obtaining the necessary partial sequences, raw data files (trace files exhibiting forward and reverse sequence information) were procured from each sequenced sample. DNA Baser software version 5.15 was used to assemble the trace files (.ABI files) that were obtained from the sequencer to create the contigs. The contigs were stored in fasta format for further bioinformatic analysis40,41,42,43,44,45. The bacterial source of the sequence was identified by aligning it against sequences exhibiting the maximum identity score from the NCBI-GenBank Database46. For comparative analysis, the BLASTn47 was used. BLASTn-generated hits of the recorded sequences exhibiting < 95% similarity were considered poor quality and were excluded from the study48.
Molecular identification and phylogenetic analysis
Phylogenetic analysis plays a chief role in revealing the systematic relationships among diacritic species, especially those that fall within novel microcosm lineages. The topological similarities among phylogenetic trees can be projected by employing specific algorithms that incorporate the nodal structures of a tree and construct a multidimensional model. These algorithms are further applied to quantify the discrepancies between subtrees49,50. The phylogenetic trees were constructed by formulating a taxonomic list that included the information on the procured 16S rRNA sequences and similarity search-driven sequences via BLASTn. The highly similar sequences were subsequently aligned with each other in multiple sequence alignment (MSA) format by utilizing the Muscle algorithm51,52,53. The Neighbor-Joining (NJ) method was used for the database set, which included the unweighted pair-group method with arithmetic averaging (UPGMA) and the maximum likelihood model for tree construction. The bootstrap test results were aggregated after 1000 replicates for all taxa. Molecular Evolutionary Genetics Analysis version XI (MEGA-XI) software was used for conducting the evolutionary analysis54,55.
Optimization of media components to produce NPs
The media optimization was performed by preparing the inoculum as mentioned above. The antagonistic activity was calibrated by the agar well diffusion method. The different parameters were optimized using a one-variable-at-a-time (OVAT) approach to obtain a substantial result56.
Effect of different media
To enhance the yield of the NPs and analyze their antagonistic activity, different liquid media cultures were used, such as, Starch Inorganic Salt broth (SIS), Yeast Malt Dextrose extract broth (YMD), Starch Casein broth (SC), Tryptone Yeast extract broth (TYE), Glycerol-Asparagine broth (GA), Glucose Soybean Meal broth (GSB), Oat-Meal broth (OM) and Tyrosine broth (TB). The initial pH of all the cultures was adjusted to 7.0. Each culture medium was incubated at 37 °C for 7 days at 150 rpm with 1% (v/v) inoculum56,57.
Effect of incubation pH
To determine the optimal pH for maximum antagonistic activity, a 5 mL aliquot of spore suspension of the Streptomyces peucetius strain was inoculated into a 250 mL flask containing 100 mL of SC medium at different pH values (3–11). Each culture medium was incubated at 37 °C for 7 days at 150 rpm36,57,58.
Effect of the incubation temperature
To determine the optimal incubation temperature for maximum antagonistic activity, a 5 mL aliquot of spore suspension of the Streptomyces peucetius strain was inoculated into a 250 mL flask containing 100 mL of SC medium at various temperatures (28–51 °C). Each culture medium was incubated at 37 °C for 7 days at 150 rpm36,57,58.
Effect of the incubation period
To determine the optimal incubation period for maximum antagonistic activity, a 5 mL aliquot of spore suspension of the Streptomyces peucetius strain was inoculated into a 250 mL flask containing 100 mL of SC medium and incubated for 1–14 days under constant conditions (pH 7, 150 rpm, 37 °C). 5 mL of culture was taken aseptically at 24-hour intervals over the stipulated period36,57,58.
Effect of carbon and nitrogen source and their concentrations
To determine the optimal carbon source for maximum antagonistic activity, a 5 mL aliquot of spore suspension of the Streptomyces peucetius strain was inoculated into a 250 mL flask containing 100 mL of SC medium. The cultures were supplemented with 1% (w/v) of different carbon sources: rhamnose, sucrose, D-glucose, maltose, inositol, starch, lactose, mannitol, xylose, D-fructose, or D-galactose. Consecutively, the cultures were supplemented with 0.4% (w/v) of different nitrogen sources: ammonium sulfate, casein, sodium nitrate, peptone, L-asparagine, yeast extract, potassium nitrate, urea, beef extract, and tryptone. Subsequently, the best carbon and nitrogen supplies were regulated independently at different concentrations to yield the maximum antagonistic activity. Each culture medium was incubated at 37 °C for 7 days at 150 rpm36,57,58.
Analysis and extraction of the optimized NPs
At the end of each optimization step, 5 mL of the culture was taken aseptically. Each culture broth was centrifuged at 5000 rpm for 10 min to separate the biomass. The supernatants were filtered via Millipore filter, then transferred aseptically into a conical flask and stored at 4 °C. Consequently, solvent extraction of secondary metabolites was conducted using ethyl acetate59.
Dry weight of NPs
Extracts were utilized to analyze NP’s dry weight, purity, and concentration. The initial wet weight of the extract was determined by meticulously weighing the designated sample with an analytical balance. The extract was dried in an oven at 80 °C to evaporate the moisture in a 24-hour cycle. After drying, the samples were re-weighed to calculate the final dry weight60,61. The following calculations (Eq. i and Eq. ii) were conducted to evaluate the amount of water removed during the process:
$$\begin{aligned}Final\:dry\:weight\: of\: NP \hspace{0.17em}=&\hspace{0.17em} \,initial\:wet\:weight\:of\:petri\:plate\:-\\ & \qquad \qquad \quad \, weight\:with\:water\:removed\end{aligned}$$
(i)
$$\:Dry\: weight\: of\: NP \:\left({\%}\right)=\:\frac{Final\:dry\:weight\:}{Initital\:wet\:weight}\:\mathsf{X}\:100\:\:$$
(ii)
Statistical optimization to produce NPs
Statistical optimization of the medium components was performed to obtain the maximum yield of NPs from the bacterial strains. The study was conducted in three phases: Plackett–Burman (PB) design, followed by a response surface methodology (RSM) method in conjunction with a Box–Behnken design (BBD), and lastly an artificial neural network design (ANN). The responses were expressed as their ZOI. The investigation was carried out in triplicate. The findings were expressed as mean ± standard error mean (SEM).
Plackett–Burman design (PBD)
Plackett–Burman design (PBD), a two-level factorial design method, was used to identify the optimum medium components that significantly affect the production of natural therapeutic products62. The Plackett–Burman experimental design is formally based on a first-order regression model that employs the following formula (Eq. iii):
$$\:{Y=\beta}_{0}+{\sum}^{k}{\beta}_{i}{\chi}_{i}$$
(iii)
where Y is the response variable, β0 denotes the model intercept, β denotes the linear coefficient, \({\chi}_{i}\) denotes the independent variables in the coded format, and k denotes the number of variables involved.
The model designates no integration among factors. It was primarily utilized for screening and evaluating the factors that strongly influence the production of NPs from a pool of available candidates63. Using the deductions of OVAT, Inositol and L-asparagine were found to be excellent carbon and nitrogen sources, respectively, to produce NPs64,65,66. The screened carbon and nitrogen sources were evaluated along with other aggregates for PBD optimization experiments. In total, ten independent variables were used: three physical — pH, temperature, and inoculum period, and seven nutritional — inositol, starch, L-asparagine, yeast extract, K2HPO4, FeSO4.7H2O, and ZnSO4.7H20 (Table 1). The experiments were designed using R programming version 4.3.167. The investigations were determined at two different levels: low (−1) and high (+ 1)64,68,69,70,71.
Box‒Behnken design (BBD)
The investigations from the PBD were utilized for executing the response surface methodology (RSM) experimental model. The RSM model is a compilation of mathematical and statistical techniques that revolve around fitting the polynomial equation of the experimental data. Following the preliminary range of the extraction variables, a three-level, three-full-factor design (TFFD) Box‒Behnken design (BBD) experimental model was devised for determining the finest combination of extraction variables72,73.
Sixteen experimental runs of the BBD were performed to assess the three independent variables – pH (4, 7, and 10), temperature (10, 30, and 60), and inositol concentration (2, 4, and 6) at three distinct coded levels; low (−1), medium (0) and high (+ 1). The significance level of each variable was established based on data from preliminary studies (Table 2).
To predict the optimal point, a second-order polynomial equation was fitted, determining the correlation between the independent variables and the NPs by the bacterial isolates (Eq. iv):
$$\:Y={\beta}_{0}+\:\:\sum\nolimits_{i=1}{\beta}_{ii}{X}_{i}^{2}\:+\:\sum\nolimits_{i=1}^{n}\sum\nolimits_{j=i+1}^{n}{\beta}_{ij}{X}_{i}{X}_{j}\:+\varepsilon\:\:\:\:\:\:\:$$
(iv)
where Y is the response variable; β0, βi, βii, and βij denote the regression coefficients for the intercept, linear, quadratic, and interaction terms, respectively; Xi and Xj denote the independent variables (i ≠ j). ε signifies the random error or discrepancies between the predicted and measured values. R programming version 4.3.1 was used for conducting the regression analysis of the experimental model74,75,76,77.
Statistical analysis of the data
The data regarding the production of NPs by the bacterial isolates were subjected to evaluate the t-value, p-value, and other statistical determinants (Eqs. v-vii). An analysis of variance (ANOVA) was conducted to evaluate the model’s statistical significance, impact, and interactions of the independent variables. The goodness and lack of fit of all the model equations were expressed by the coefficient of determination R2 and Adjusted-R2. The statistical and regression coefficient significance were determined by employing Fisher’s test at a probability value (p) ≤ 0.05. The optimal extraction conditions were assessed via linear and multiple regression model analysis using graphical presentations: pareto (2D), response surface (3D), and cube (3D) plots by employing the R programming version 4.3.164,66,67,68,69,70,71,72,73,74,75,76,77. Additionally, statistical heatmaps were constructed using Python programming version 3.12.478.
$$\:\text{M}\text{S}\text{E}\:=\:\:\:\frac{1}{n}\:\sum\nolimits_{i=1}^{n}(y-\widehat{y})^2$$
(v)
$$\:\text{R}\text{M}\text{S}\text{E}\:=\:\sqrt{\frac{1}{n}}\sum\nolimits_{i=1}^{n}(y-\widehat{y})^2\:$$
(vi)
$$\:\text{M}\text{A}\text{E}\:=\:\:\frac{1}{n}\sum\nolimits_{i=1}^{n}\left|\widehat{y}-y\right|\:$$
(vii)
where Mean Squared Error (MSE), Root Mean Squared Error (RMSE), and Mean Absolute Error (MAE). n denotes the number of samples, y signifies the anticipated values of the response variable, and \(\:\widehat{y}\) signifies the predicted values of the response variable.
Artificial neural network (ANN)
An Artificial Neural Network (ANN) configuration was employed for optimizing and predicting the target variables based on the input features. Python version 3.12.478 and PyTorch version 2.6.079 were employed for constructing the neural network model. The ANN model was trained by utilizing the independent variables from the RSM model80,81. To enhance the prediction capability, the dataset was pre-processed before conducting the model training. The Standard-Scaler module from scikit-learn82 was employed for normalizing and standardizing the input features (pH, temperature, and inositol concentration) and target variables (ZOI). Equitable assessment of the model was facilitated by classifying the dataset: training (70%), testing (15%), and validation (15%)83.
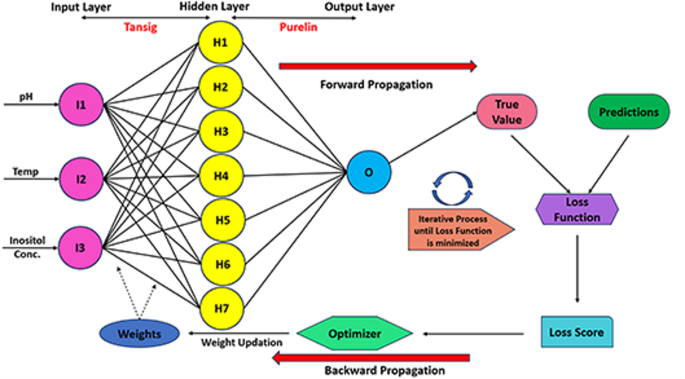
The ANN model skeleton constituted a three-layer feedforward neural network framework. The model topology exhibited an input layer with three neurons, the hidden layer with adaptive neurons (between three to seven), and the output layer with one neuron. The hidden layer deployed a hyperbolic tangent (Tansig) activation function, while the output layer incorporated a pure linear activation function (Purelin). The graphical working representation of the ANN model has been illustrated in Fig. 2. The predicted values of the ANN model were determined by calculating the output from each neuron in the hidden layer and by summing each neuron and its weight (Eqs. viii-ix)84,85:
$$\:{H}_{i}\:=tanh\left(0.5\right(\sum\nolimits_{m=1}^{N_i}\left({w}_{i.m}{x}_{m}\right)+\:{b}_{i}\left)\right)$$
(viii)
$$\:Y_{ANN}=\sum\nolimits_{i=1}^{N_h}{{w}}_{2,{i}}{{H}}_{{i}}\:+\:{{b}}_{{h}}$$
(ix)
where Hi is the hidden layer, Xm, is the value for the input variable, bi and bh are the biases in the input and hidden layers, respectively.
The model training was executed by the implementation of the Levenberg-Marquardt (LM) algorithm, using the Limited-memory Broyden-Fletcher-Goldfarb-Shanno (LBFGS) optimizer. L2 regularization was used to improve the model’s generalizability and mitigate the model’s overfitting capability. The MSE loss function was applied as the parameter for evaluating the performance of the model and calculating the difference between the experimental and predicted values. The training of the model effectively terminated when the generalization procedure ceased to improve due to a rise in the validation MSE values. Sensitivity analysis was conducted based on the Garson equation86 (Eq. x). The final model was subjected to numerous statistical measures87,88.
$$\:{I}_{j}=\frac{\sum\:_{m=1}^{m={N}_{h}}\left\{\:\right(\:\frac{\left|{W}_{jm}^{jh}\right|}{\sum\:_{k=1}^{{N}_{i}}\left|{W}_{km}^{ih}\right|})\:\times\:\:|{W}_{mn}^{ho}\left|\right\}}{\sum\:_{k=1}^{k={N}_{i}}\{\:\sum\:_{m=1}^{m={N}_{i}}(\:\frac{\left|{W}_{km}^{jh}\right|}{\sum\:_{k=1}^{{N}_{i}}\left|{W}_{km}^{h}\right|})\:\times\:\:|{W}_{mn}^{ho}\left|\right\}}\:\times\:\:100$$
(x)
where Ij is the relative importance (%) for jth parameter of model output. Ni and Nh are the input and hidden layer neurons, respectively. W is the connection weight, while i, h, and o are the input, hidden, and output layers, respectively. The variables k, m, and n are the neuron numbers in the input, hidden, and output layers, respectively.
Validation of the ANN models
The validation analysis of the ANN model is a crucial step in evaluating the model’s robustness, generalization, and reliability for varied datasets. The focus was to assess the ANN model’s performance with the concealed data. It further inhibited the underfitting or overfitting process. Therefore, k-fold Cross-Validation (CV) was employed to determine the model’s performance. Python version 3.12.4 and PyTorch version 2.6.0 were employed to conduct the validation analysis54,89.
Determination of the 16S rRNA structure
The secondary structures of 16S rRNA were constructed by applying free energy minimization techniques. These free energies were procured from experimental measurements of RNA structural element stability25,90,91,92,93,94,95. The secondary structure determination of the 16S rRNA was performed by utilizing the Unified Nucleic Acid Folding and Hybridization Package (UNAfold) with the MFold server. This server implements the Zuker-Stiegler algorithm for calibrating the minimum free-energy structure depending on the nearest neighbor model. The empirical estimates of the thermodynamic properties and the sub-optimal structures having a certain degree of free energy were determined for the rRNA structures. The mole fractions of the structures as a function of UV absorbance (A260), heat capacity (Cp), temperature, and complete melting profiles for melting simulations were also calculated96,97,98,99,100.