Tulsi Therapeutics, a biotech startup from Hyderabad, has reversed chronic liver failure in animal trials using Tulsi-28X, a first-in-class stem cell–exosome therapy. The innovation showed 100% survival and fibrosis reversal, setting the stage for human clinical trials.
Updated On – 24 July 2025, 02:01 PM
Hyderabad: In a potential game-changer for people suffering from chronic liver disease, Tulsi Therapeutics, a startup incubated at ASPIRE-BioNEST, University of Hyderabad (UoH), has successfully reversed chronic liver failure in preclinical trials.
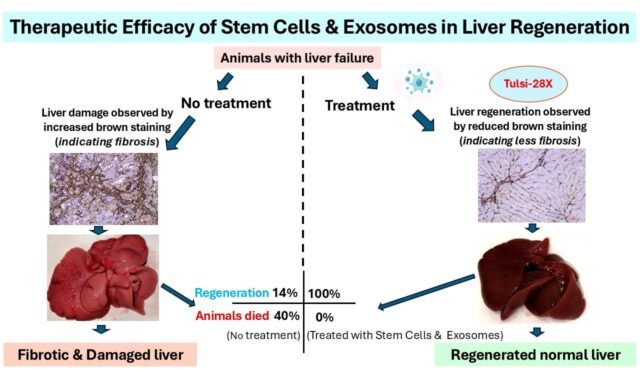
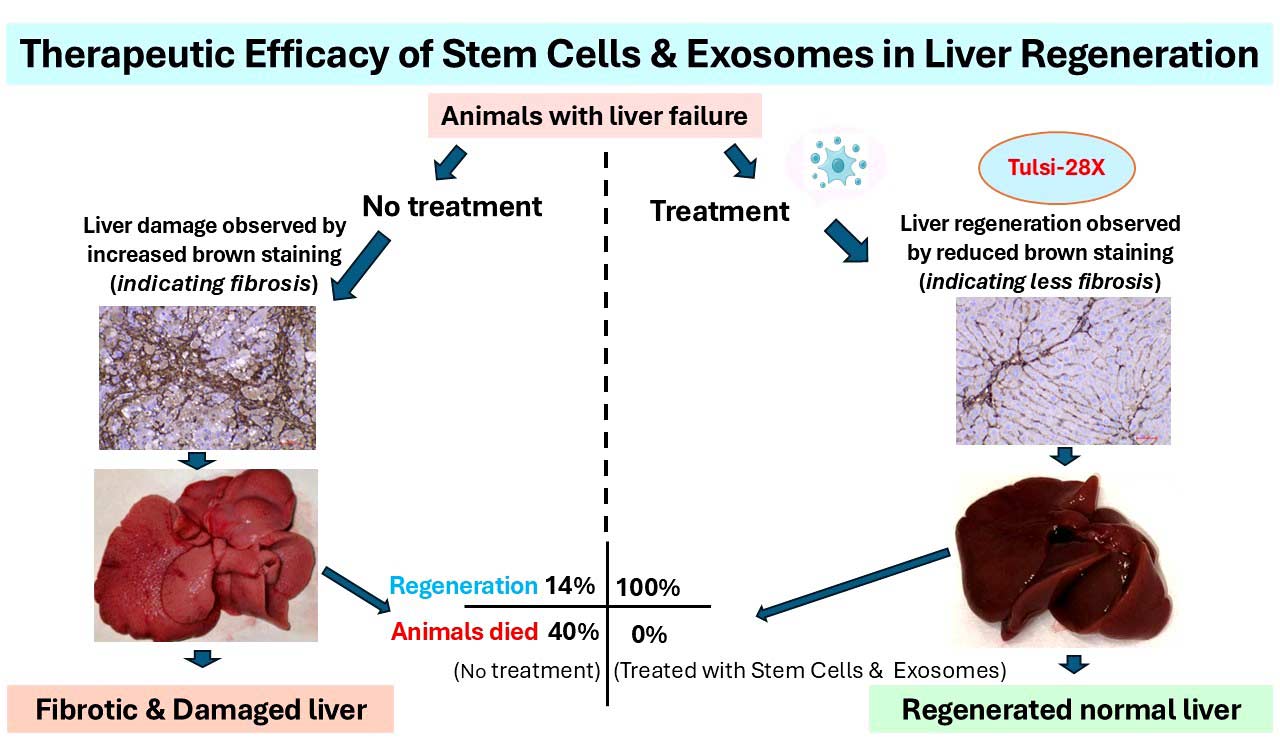
Using the novel stem cell–exosome combination therapy, the start-up demonstrated 100 percent of the animals treated with Tulsi-28X, an investigational product, showed reversal of liver fibrosis (indicating liver regeneration) resulting in zero deaths, compared to only 14 percent reversal and 43 percent deaths in the untreated control group.
The preclinical trial was conducted in collaboration with global experts including Dr. Naga Chalasani (Indiana University, USA) and Dr. Ajay Duseja (PGIMER, Chandigarh). According to the start-up, Tulsi Therapeutics is also the world’s first biotech company developing dual stem cell–exosome biologics. Tulsi-28X works by secreting regenerative proteins and growth factors, stimulating the repair of diseased liver tissue.
The investigational product, Tulsi-28X, is a first-in-class regenerative therapy derived from Wharton’s Jelly mesenchymal stem cells and their native exosomes—a combination never before tested in any animal model worldwide. While conceptualized in the United States, the platform was entirely developed in India through three years of intensive research at ASPIRE-BioNEST.
“This is a significant milestone for India’s biotech industry. While human trials are the next challenge, this study opens doors to a new class of biologics in liver disease,” said Dr. Sairam Atluri, Founder & CEO Tulsi Therapeutics.
Key results were presented at the prestigious AASLD 2024 Liver Conference in San Diego and accepted for publication in the Journal of Regenerative Medicine.
“We are committed to developing world-class yet affordable regenerative solutions. Our next step is to take Tulsi-28X into human clinical trials in collaboration with Nizam’s Institute of Medical Sciences (NIMS),” said Dr. Ravi Bonthala, Chief Scientific Officer Tulsi Therapeutics.
Chronic liver failure remains a serious public health concern in India, contributing to nearly 20 percent of global liver-related deaths. With transplantation being the only current treatment, Tulsi-28X represents a potential paradigm shift.
“This milestone highlights how India’s innovation ecosystem—anchored by institutions like the UoH and enabled by BIRAC and incubators like ASPIRE-BioNEST—is capable of delivering breakthrough global biotech products,” said Prof. BJ Rao, Vice Chancellor, UoH.